Junji Nakamura Lab.

Blueprint for a Methanol Society: Toward Carbon-Neutrality
01
CO2の排出源(世界)は、発電所 > 鉄鋼業 > セメント業 > 化学工業の順であり、 合わせると65%になります。
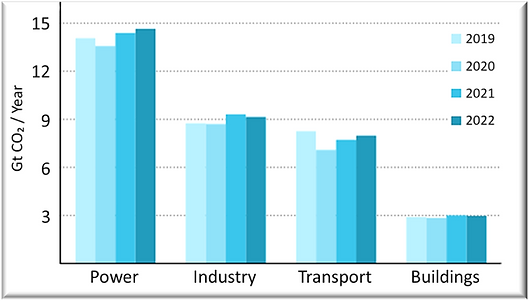
Power plants emit most CO₂.
-
Industry sector includes steel, cement, chemicals, etc.
-
Power & Industry account for 65% of all CO₂ emissions.
-
Cheap coal and oil are mainly used, but these must be replaced with renewable energy sources such as solar and wind power.
Problems with solar power generation

Renewable energy is essential!

Daytime only solar cells

People tend to discuss electric vehicles,
which is not the main issue.
Supplying electricity in a way that responds quickly to demand is important.
Artificial fuels are necessary.
Batteries are not enough.
02
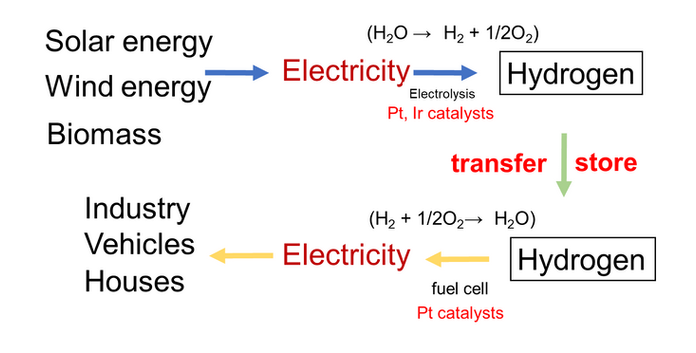
Hydrogen Society (Artificial fuel)
Hydrogen is not an “energy source,” but an energy carrier and energy storage.
03
Why is the introduction of artificial fuels so slow?
Fossil fuels are cheap!
-
Oil is cheap because humans simply dig up oil and use it like water.
-
Isn't something wrong?


Spring water (~1 USD / L)
& Gasoline (~1 USD / L)
Artificial fuels are expensive!
This is because the production of artificial fuels requires fuel for the manufacturing process.
Liquid H2
Ammonia
Methanol
H₂O → H₂ + ½ O₂ using electricity and expensive Ir catalysts, Energy is required to cool at –253°C. New technology is necessary for converting H₂ to energy.
N₂ + 3 H₂ → 2 NH₃ at 100~300 atm and 400~500 ℃. Energy is required to separate N₂ from air. Special technology is necessary for converting NH₂ to energy.
CO₂ + 3 H₂ → CH₃OH + H₂O at 30~50 atm and 250 ℃ using Cu/ZnO.
The cost of H₂ is still high.
04
The most important discussion is which artificial fuel to use.

Liquid Hydrogen(H2)
– 253 ℃!
Advanced technology is
necessary for handling liquid H2 .

Ammonia (NH₃)
Highly toxic!
Consumes a lot of energy
Advanced technology is necessary.

Methanol (CH₃OH)
Existing Technology!
Available at drug stores
Ready to use as fuels
05
“Proposal for Methanol Society
—Toward a Carbon-Neutral Society”

By Junji Nakamura and Masayoshi Ishida
Part I: What Are the Problems?
1. Global Warming and CO₂ Emissions
2. Energy Problems
3. Issues in Industry
4. Political Issues

Part II: What Path Should We Take? The Methanol Society
5. Why a Methanol Society?
6. Methanol as an Energy Source
7. Chemical Uses of Methanol
8. Progress Towards a Methanol Society


Part III: What Should We Do? Business, R&D, Policy, Philosophy, Education
9. Roadmap and Business Development of the Methanol Society
10. Technologies Expected in a Methanol Society
11. Catalytic Chemistry of Methanol
12. Future Perspectives
06
The energy and industrial uses of methanol have already been proposed by Nobel Prize winners in 2005.


07
Methanol synthesis from CO2 has been widely studied since the 1980’s.
Japan's national project to produce methanol from CO2 began in 1993 (RITE).

The CuZn alloy active site was
proposed in 1997.



08
A methanol society is promising.


Advantage ① : A society where CH₃OH and CO₂ are recycled

Advantage ③: Methanol synthesis from CO₂ has already been commercialized.



Advantage ② : Already used as fuel, easy and safe to use, basic technology is established

Advantage ④: Can be converted to chemical raw materials
09
True innovation is cheap technology that can be used in developing countries!
Methanol synthesis conditions are relatively mild.


Waste wood & waste
plastics can be used.
Reaction temperature: 250℃, Reaction Pressure 30-80 atmosphere

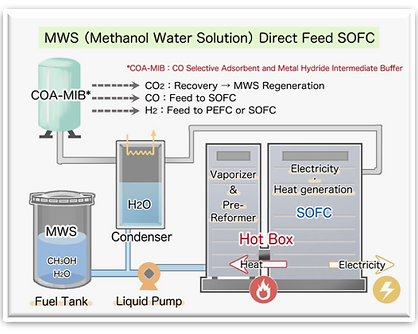
Engine with CO2 recycling
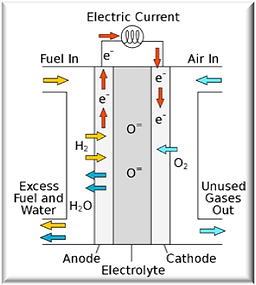
Fuel Cell (SOFC)